TRENDING TAGS :
AstraZeneca may have used 'Old Data' during vaccine trial: US Agency
According to the US National Institute of Allergy and Infectious Disease, the Data Safety Monitoring Board has raised concerns about AstraZeneca's data.
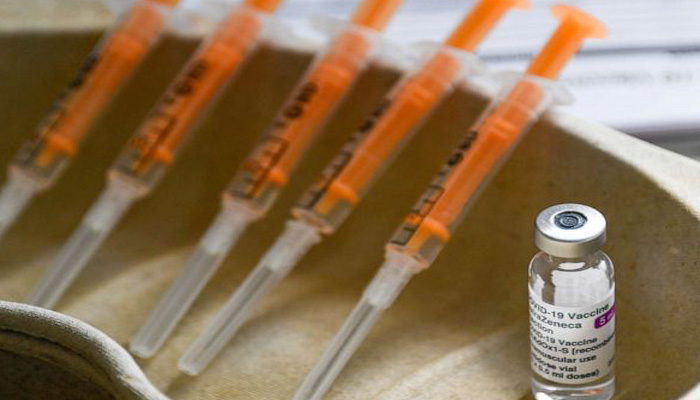
New Delhi: The Corona vaccine of the British-Swedish pharmaceutical company AstraZeneca has once again come under suspicion. The US Health Agency suspects that the company used old data during the trial. Let us know that the day before, AstraZeneca has announced the results of the vaccine trial in America. Under this, it has been said that vaccine is 79% effective.
American Agency suspects the use of Astrazeneca:
According to the US National Institute of Allergy and Infectious Disease, the Data Safety Monitoring Board has raised concerns about AstraZeneca's data. The board suspects that AstraZeneca used old data during the trial. So it is difficult to tell exactly how effective this vaccine is. The final decision on the use of this vaccine in America will be made by an Advisory Committee.
ALSO READ: Gun Firing in Colorado supermarket; 10 people died
A large trial of the Oxford AstraZeneca vaccine in the US and South American countries has revealed that the vaccine is 79 percent effective at preventing Covid-19 and up to 100 percent effective at preventing the disease from becoming serious.
The third phase of the vaccine, developed by Oxford University and the AstraZeneca Company, was studied in the US, Chile and Peru, confirming its "safe and effective".
Earlier, vaccines were tested in Britain, Brazil and South Africa. This vaccine is also being produced by the Serum Institute of India. The vaccine was seen to be equally effective on people of all ages and communities and it was 80 percent effective on persons above 65 years of age.
ALSO READ: WHO confirms ‘AstraZeneca is safe’; EU Countries to resume its use
Andrew Pollard, a professor at the University of Oxford and principal investigator for vaccines, said, "The results are good news because it shows the vaccine's impact." It also looks equally effective in the outcome of the Oxford trial. "
Stay tuned with the newstrack to get fastest updates. Click @englishnewstrack to follow us on Facebook and @newstrackmedia to follow on Twitter.