TRENDING TAGS :
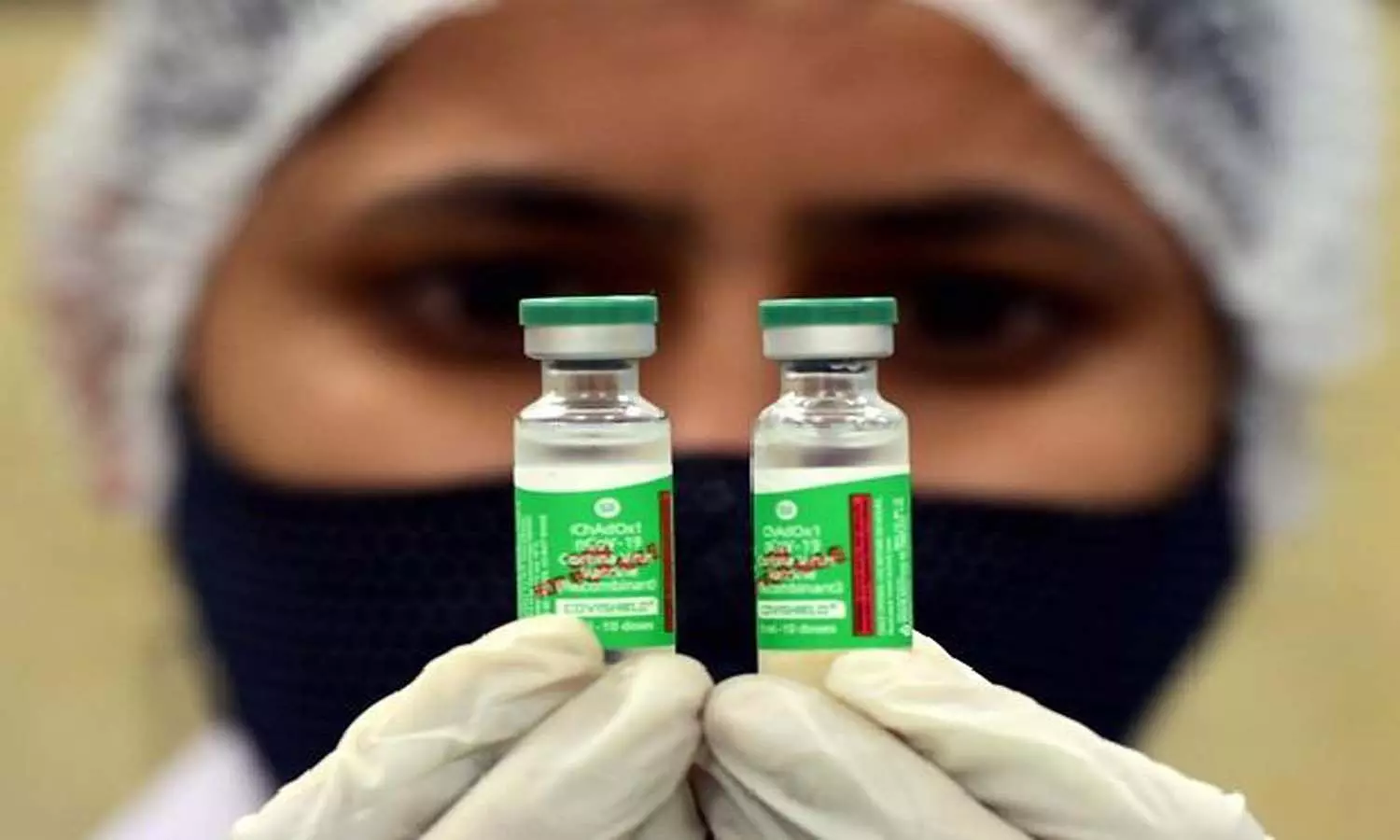
26 'potential' cases of bleeding, clotting after Covishield vaccine in India
The AEFI Committee said it had studied 498 (of 700) "serious and severe events" and found that only 26 had been reported as "potential thromboembolic events".
The health ministry on Monday said that a government panel on AEFI (adverse events following immunisation) has found 26 potential cases of bleeding and clotting after administration of the Covid-19 vaccine, Covishield.
AEFI committee's report reveals bleeding, clotting cases
The AEFI Committee said it had studied 498 (of 700) "serious and severe events" and found that only 26 had been reported as "potential thromboembolic events", referring to the potentially fatal formation of a blood clot that could break loose and be carried by the blood stream to block another vessel.
"There is a very minuscule but definitive risk of thromboembolic events" after being vaccinated, the data showed.
The panel also noted that Covishield - the AstraZeneca-Oxford University Covid-19vaccine linked by some studies to clotting issues - reported fewer than 0.61 cases per million doses administered.
Covishield:
The panel said that as of 3 April, 75,435,381 vaccine doses had been administered (Covishield – 68,650,819; Covaxin – 6,784,562). Of these, 65,944,106 were first doses and 9,491,275 second dose.
Since the coronavirus vaccination drive was initiated – over 23,000 adverse events were reported through the CO-WIN platform reported from 684 of the 753 districts of the country. Of these, only 700 cases (@ 9.3 cases /million doses administered) were reported to be serious and severe nature, it added.
No potential thromboembolic events after administering Covaxin
The panel also said Covaxin - the vaccine developed by Bharat Biotech - reported "no potential thromboembolic events" following the administering of the drug.
"It is important to know that thromboembolic events keep occurring in the general population... background and scientific literature suggests this risk is almost 70% less in persons of South and South East Asian descent in comparison to those from European descent," the panel said.
The health ministry will issue advisories to Healthcare Workers and Vaccine Beneficiaries to encourage people to be aware of suspected thromboembolic symptoms occurring within 20 days after receiving any Covid vaccine (particularly Covishield) and report preferably to the health facility where vaccine was administered:
breathlessness;
pain in chest;
pain in limbs/pain on pressing limbs or swelling in limbs (arm or calf);
multiple, pinhead size red spots or bruising of skin in an area beyond the injection site;
persistent abdominal pain with or without vomiting;
seizures in the absence of previous history of seizures with or without vomiting;
severe and persistent headache with or without vomiting (in the absence of previous history of migraine or chronic headache);
weakness/paralysis of limbs or any particular side or part of the body (including face);
persistent vomiting without any obvious reason;
blurred vision or pain in eyes or having double vision;
change in mental status or having confusion or depressed level of consciousness
Any other symptom or health condition which is of concern to the recipient or the family
In a statement, the government further said that Covishield continues to have a definite positive benefit-risk profile with tremendous potential to prevent infections and reduce deaths due to Covid-19 across the world and in India.
Over 13.4 crore doses of Covishield vaccine have been administered as of 27 April in India, it added.
Stay tuned with the newstrack to get fastest updates. Click @englishnewstrack to follow us on Facebook and @newstrackmedia to follow on Twitter.